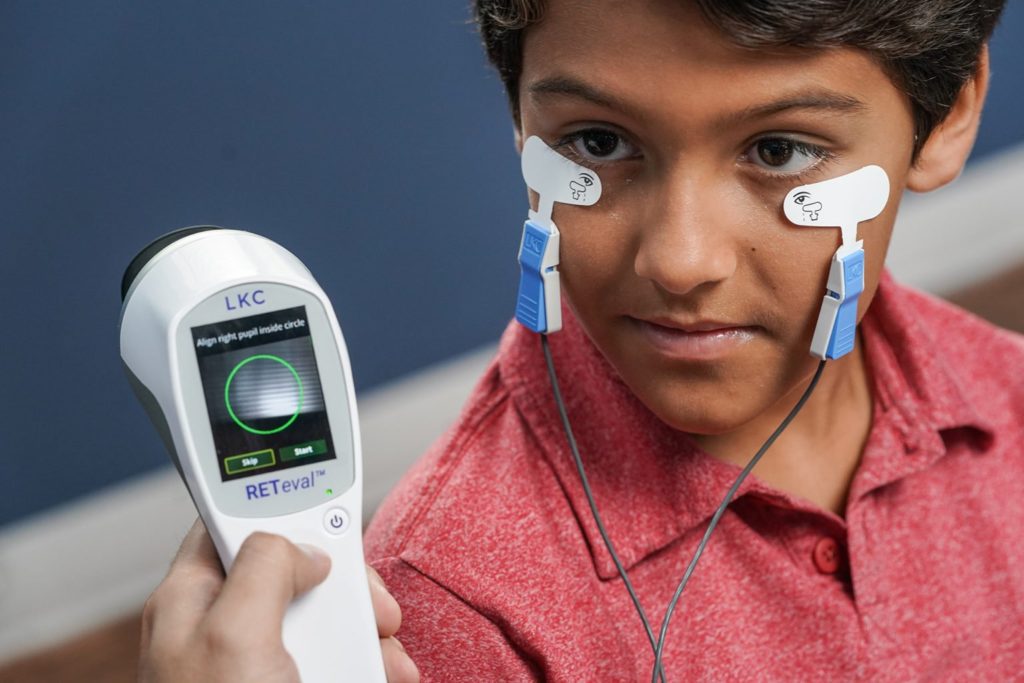
For 45+ years, LKC Technologies has been demystifying visual electrophysiology, helping clinicians in the diagnosis and management of ischemic, optic nerve, and other retinal dystrophies.
The company was founded in 1975 by Jerome Leight, Sigmund Krassowsky, and Frank Chen; hence, the initials LKC. Its first device was a telemetry in-hospital monitoring system for cardiac patients. In early 1976, LKC was introduced to a new device for the diagnosis of retina and optic nerve disease. This was the infancy of the Visual Electrodiagnostic Testing System.
Since 1976, LKC Technologies established itself as the industry leader in the manufacture of visual electrophysiology products.

At LKC, we aim to preserve vision by offering technology that makes functional retinal assessments accessible everywhere, for everyone.
As a global organization, LKC’s committed representatives are driven to make a difference through innovative technology and quality customer service.

![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
