Article
Optometry’s Community
April 2024
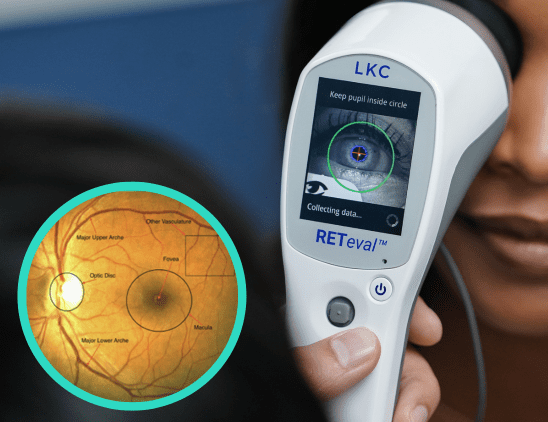
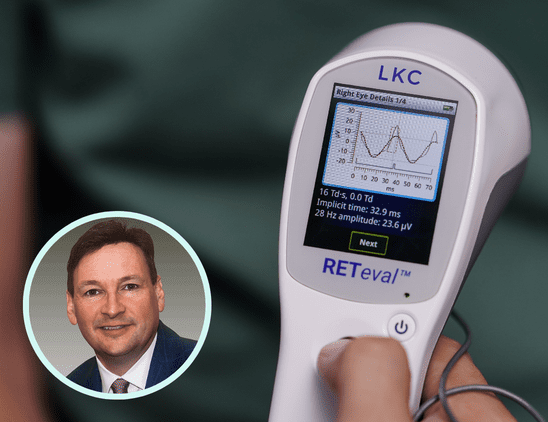
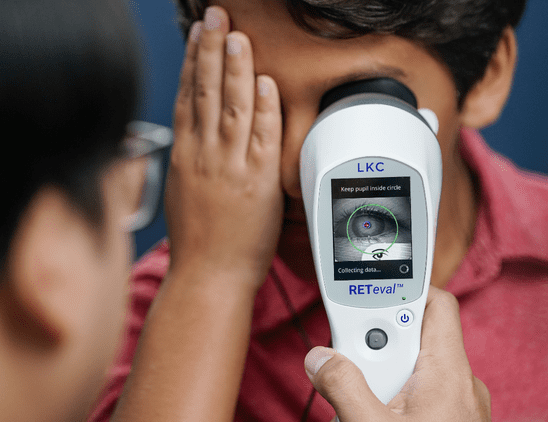
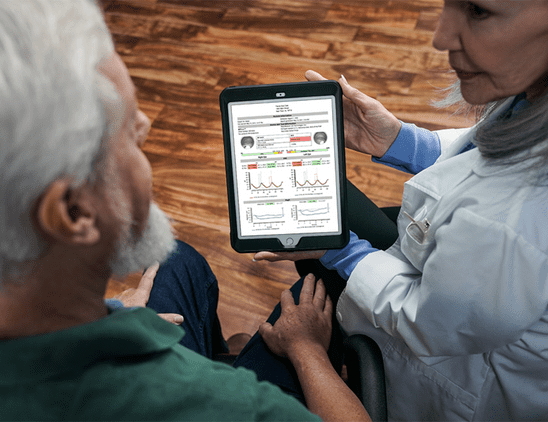
ERG detects functional stress so that we can anticipate structural damage. In studies comparing ERG and imaging for diabetic retinopathy, ERG was better at predicting which patients would need intervention.