Article
Review of Optometry
March 2023
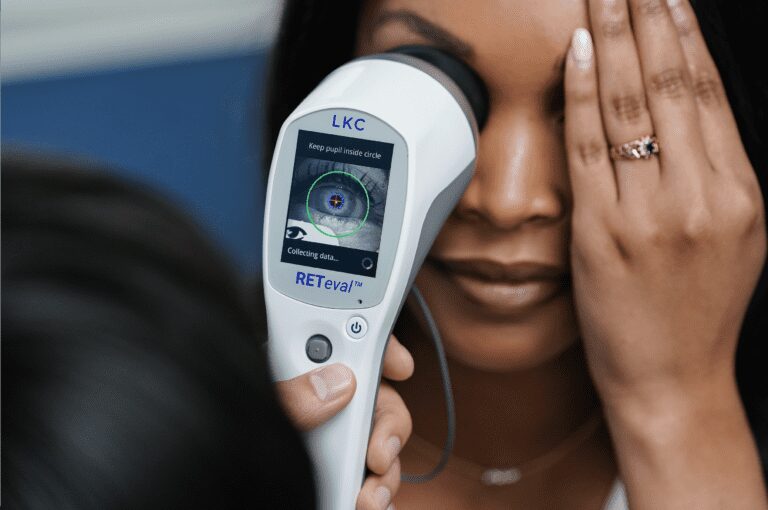
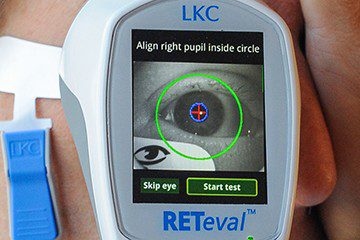
Leading optometrists use functional testing as an important complement to structural tests. Read the March 2023 issue of Review of Optometry to find ...